

BASIC BIOLOGY OF CRYPTOSPORIDIUM
(Division of Biology, Kansas
State University)
New book: Cryptosporidium: From
Molecules to Disease
Released: 2003. ISBN 0-444-51351-5. $139.00 USD
Editors: Thompson, R.C.A., Armson, A., and Ryan, U.M.
Publisher: Elsevier Science B.V.
Contents: Proceedings of the meeting
entitled "Cryptosporidium: from
Molecules to Disease." 07-12 October 2001, Fremantle, Western Australia.
Full papers and extended abstracts.
Section 1) Cryptosporidiosis-aetiology, infectiviy and pathogenesis;
Section 2) Epidemiology and species differentiation; Section 3) Viability
and infectivity; Section 4) Cryptosporidium and the environment;
Section 5) Cryptosporidium - chemotherapy; Section 6) Synthesis
(summary).
Excellent source book:
Cryptosporidium and
Cryptosporidiosis
Released: January, 1997. ISBN: 0-8493-7695-5. $89.95 USD
Editor: Dr. Ron Fayer, USDA, Beltsville, MD.
Publisher: CRC Press, Boca Raton. 288 pp.
Contents: Chap. 1) General Biology by R Fayer, CA Speer, & JP
Dubey; Chap. 2) Diagnosis by MJ Arrowood; Chap. 3) Human and Animal
Epidemiology by DP Casemore, SE Wright, & RL Coop; Chap. 4) Waterborne
Crypto: incidence, outbreaks, and treatment strategies by JB Rose, JT
Lisle, & M LeChevallier; Chap. 5) Prophylaxis and Chemotherapy (human and
animal) by BL Blagburn & R Soave; Chap. 6) Immunology by MW Riggs; Chap. 7)
Biochemistry by M Tilley & SJ Upton; Chap. 8) In vitro Cultivation by SJ
Upton; Chap. 9) Laboratory Models by DS Lindsay; Chap. 10) Molecular
Biology of Cryptosporidium by MC Jenkins & C Peterson.
INTRODUCTION. Members of the genus Cryptosporidium infect epithelial surfaces, especially those along the gut, and can be found in a wide range of vertebrates, including humans. The type species, C. muris Tyzzer, 1907, infects the gastric glands of laboratory rodents and several other mammalian species (1907, Proc Soc Exp Biol Med 5: 12-13; 1910, J Med Res 23: 487-509; 1989, Parasitol Res 75: 218-222). It is only rarely known to infect humans but a handful of case studies have been reported (2000, Am J Trop Med Hyg 62: 70-72; 2003, Emerg Inf Dis 9: 1174-1176). A second species, C. parvum Tyzzer, 1912, infects the small intestine of an unusually wide range of mammals, including humans, and is the zoonotic species responsible for approximately one-half of human cryptosporidiosis (1912, Arch Protistenkd 26: 394-412; 2000, Int J Parasitol 30: 1305-1322). A relatively newly discovered species, C. hominis Morgan-Ryan et. al., is responsible for the other one-half of human infections (2002, J Euk Microbiol 49: 433-440). This species is morphologically identical to C. parvum, but is more difficult to study due to lack of animal models.
Cryptosporidium translates into "hidden spore" (or perhaps more specifically in latin "underground spore"). Tyzzer explains the name on page 504 of his 1910 (J Med Res 23: 487-509) paper as follows: "...the intention being to signify that it is a sporozoon in which spores are indistinguishable or absent in the oocyst..." Anotherwards, Tyzzer was differentiating the genus Cryptosporidium from coccidian oocysts because it was structurally distinct. Coccidian oocysts usually have sporozoites encased in smaller spores termed "sporocysts," notably absent in this new organism.
In recent years, other species of Cryptosporidium have been found in humans. As mentioned above, C. hominis (formerly C. parvum genotype I), is relatively specific for humans and is morphologically indistinguishable from C. parvum (2002, J Eukaryot Microbiol 49: 433-440). This parasite is responsible for many human infections and outbreaks. Other species of lesser importance in humans include C. meleagridis, C. canis, and C. felis (2000, Appl Environ Microbiol 66: 2220-2223; 2001, J Parasitol 87: 1415-1422; 2001, J Infect 42: 243-250; 2002, Emerg Inf Dis 8: 85-86; 2003, Infect Immun 71: 1828-1832).
The taxonomic status of the genus Cryptosporidium is becoming increasingly clear. It has traditionally been aligned with the coccidia. However, recent molecular studies (i.e. 1999, Parasitol Res 85: 899-904) have shown that members of the genus are actually more closely related to the gregarines than to eimerians or even the malaria. Actually, the genus is very divergent from the gregarines as well. The "traditional" taxonomic position of the genus Cryptosporidium is as follows, although technically it should be removed from the suborder Eimeriorina.
Cryptosporidium parvum is predominately a parasite of neonate animals. Although exceptions occur, older animals generally develop poor infections, even when unexposed previously to this parasite. Experimental laboratory infections in immunosuppressed adult animals have shown that infections build up slowly, and only occasionally progress to the level found in neonates. It is likely that one or more developmentally regulated antigen(s) along the intestinal tract are responsible for neonates, rather than adults, developing severe cryptosporidiosis. One study has suggested that this may be a 57 kDa antigen found on ileal cells of neonates that binds a 47 kDa ligand at the apical end of sporozoites (1999, Biochim Biophys Acta 1454: 165-173). Another study has reported that the CSL (rhoptry) protein binds a 85 kDa receptor on epithelial cells (2001, Inf Immun 69: 1661-1670). Humans, on the other hand, are the one host that can be infected at any time in their lives, and only previous exposure to the parasite results in either full or partial immunity to challenge infections.
LIFE-CYCLE. The life cycle of C. parvum is depicted below and begins with ingestion of the sporulated oocyst, the resistant stage found in the environment. Each oocyst contains 4 infective stages termed sporozoites, which exit from a suture located along one side of the oocyst. The preferred site of infection is the ileum, and sporozoites penetrate individual epithelial cells in this region. Parasites reside on the lumenal surface of the cells, and they were once thought to occur extracellularly. However, ultrastructural observations have clearly shown these parasites to be intracellular, enclosed by a thin layer of host cell cytoplasm. A unique, desmosome-like attachment organelle, plus accessory foldings of the parasite membranes, develop at the interface between the parasite proper and the host cell cytoplasm. This attachment organelle is sometimes referred to as the "feeder organelle." Multiple fission (=merogony; =schizogony) occurs, resulting in the formation of 8 merozoites within the meront. These meronts are termed Type I meronts and rupture open, releasing free merozoites. Once these merozoites penetrate new cells, they undergo merogony to form additional meronts. Type I merozoites are thought to be capable of recycling indefinitely and, thus, the potential exists for new Type I meronts to arise continuously.
It is thought that some Type I merozoites are somehow triggered into forming a second type of meront, the Type II meront, which contains only 4 merozoites. Once liberated, the Type II merozoites appear to form the sexual stages. Some Type II merozoites enter cells, enlarge, and form macrogametes (=macrogametocyte). Others undergo multiple fission once inside cells, forming microgametocytes containing 16 non-flagellated microgametes. Microgametes rupture from the microgametocyte and penetrate macrogametes, thus forming a zygote. A resistant oocyst wall is then formed around the zygote (the only diploid stage in the life cycle), meiosis occurs, and 4 sporozoites are formed in the process. Formation of sporozoites is termed sporogony. These oocysts are passed in the feces and into the environment. Each haploid nucleus of the sporozoite contains 8 chromosomes consisting of 10.1-10.4 million base pairs of DNA with very few introns, and the cytoplasm about 1,000 copies each of two types of encapsidated double-stranded RNA molecules representing a virus of the family Partitiviridae (2000, J Virol 74: 5788-5795).
Approximately 20% of the oocysts produced in the gut fail to form an oocyst wall and only
a series of membranes surround the developing sporozoites. These "oocysts," devoid of a
wall, are sometimes termed "thin-walled oocysts." It is believed that the resulting sporozoites
produced from thin-walled oocysts can excyst while still within the gut and infect new cells.
Thus, C. parvum appears to have two autoinfective cycles: the first by continuous
recycling of Type I meronts and the second through sporozoites rupturing from thin-walled
oocysts.
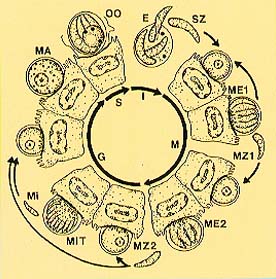
 Life cycle of Cryptosporidium parvum.
Abbreviations: (E)
Excystation (either as thick-walled oocyst from environment or via
thin-walled oocyst excysting in situ), resulting in release of 4 sporozoites
through suture in wall; (G) Gamogony; (I) Infective phase; (M) Merogony; (ME1) Type I meront
containing 8 merozoites; (ME2) Type II meront containing 4 merozoites;
(MA) Macrogamete, containing wall forming bodies;
(Mi) Microgamete; (MiT) Microgametocyte with 16 non-flagellated microgametes;
(MZ1) Type I merozoite; (MZ2) Type II merozoite; (OO) oocyst; (S)
Sporogony; (SZ) sporozoite. See 1986, J Protozool 33: 98-108.
Life cycle of Cryptosporidium parvum.
Abbreviations: (E)
Excystation (either as thick-walled oocyst from environment or via
thin-walled oocyst excysting in situ), resulting in release of 4 sporozoites
through suture in wall; (G) Gamogony; (I) Infective phase; (M) Merogony; (ME1) Type I meront
containing 8 merozoites; (ME2) Type II meront containing 4 merozoites;
(MA) Macrogamete, containing wall forming bodies;
(Mi) Microgamete; (MiT) Microgametocyte with 16 non-flagellated microgametes;
(MZ1) Type I merozoite; (MZ2) Type II merozoite; (OO) oocyst; (S)
Sporogony; (SZ) sporozoite. See 1986, J Protozool 33: 98-108.
Development of Cryptosporidium occurs more rapidly than many textbooks imply, and each generation can develop and mature in as little as 12-14 hours. Due to the rapidity of the life cycle, plus the autoinfective cycles, huge numbers of organisms can colonize the intestinal tract in several days. The ileum soon becomes crowded and secondary sites are often infected, such as the duodenum and large intestine. In immunosuppressed individuals, parasites can sometimes be found in the stomach, biliary and pancreatic ducts, and respiratory tract. Diarrhea, weight loss, and abdominal cramping are clinical signs of the disease and in immunosuppressed individuals electrolyte imbalance may occur. The prepatent period, which is the interval between infection and the first appearance of oocysts in the feces, is generally 4 days (3 days in heavy infections) in animals infected experimentally. However, in human outbreaks where lower numbers of oocysts are probably ingested, 4-6 days is probably typical. Patency, which is the length of time oocysts are shed in the feces, generally lasts 6-18 days (4-10 days of diarrhea) in immunocompetent individuals but may be prolonged in immunosuppressed patients. Some individuals shed oocysts but appear asymptomatic. The figure below depicts typical oocyst shedding in Holstein calves that were each infected orally at 4 days of age with 25 million oocysts of C. parvum. No oocysts were detected until 4 days post-infection (DPI). All feces were then collected from each calf at 24 hr intervals and numbers of oocysts quantified. As can be seen, peak oocyst production occurred 6-8 DPI and numbers produced were highly variable between the animals. In our experience, as few as 2 billion and as many as 20 billion oocysts can be collected during a single 24 hr period from calves during peak oocyst shedding. A single calf can easily produce 50 billion oocysts within a period of one week. Several recent studies have suggested Cryptosporidium spp. have gregarine-like stages, hypothetically making the life-cycle more complicated. I am totally convined that these studies are erroneous and represent yeast, fungal spores (conidia), and other forms of cell culture contamination.
EPIDEMIOLOGY. Huge numbers of
Cryptosporidium oocysts exist in the
environment. Recently, for the 101st annual meeting of the American
Society for Microbiology in Orlando, I attempted to roughly calculate how
many
oocysts cattle alone might produce per year in the U.S. The results
are truly impressive. Using the average number of 69 oocysts/g
in typical bovine manure (positive plus negative samples combined; 2000,
Environ Res A, 82: 263-271), taking into account that there were about 103
million cattle at any one time in the U.S. in the year 2000 (USDA
numbers), the fact that the average bovid produces about 25 kg of feces
per day (5.9 kg/day for calves - nearly 55 kg/day for large
Holsteins; USDA
data), and that there are 365 days per year (a no brainer), then cattle
alone produce nearly 65 quadrillion oocysts per year in the
U.S.! That's 65 with a staggering 15 zeros behind it
folks. Considering
that the U.S. has a total surface area of 9,629,091 sq km, that the total
area of an oocyst is about 18.787 square micrometers (pi x L/2 x W/2), and
that 64.851375 quadrillion oocysts are produced by cattle per year, then
bovids produce nearly 6,735 oocysts each year for every square meter
of surface area in the U.S. And that doesn't count oocysts generated by
sheep, goats, deer, swine,
horses, dogs, cats, and other animals! And, to take the trivia one step
further, consider the following. The average volume of an oocyst is
57.613 cubic micrometers (4/3 x pi x L/2 x W/2 x D/2) and there are
one trillion cubic micrometers in a cubic centimeter. Thus, the average
number of oocysts per cubic centimeter should be about 17,357,308,030
(17.4 billion). We've performed some careful density gradient
studies on oocysts in the laboratory and have found that the specific
gravity of an oocyst averages about 1.109. This means that about
15,651,314,725 oocysts make a gram. Using these numbers, and with
907,184.74 grams per ton and 64.851375 quadrillion oocysts (see above),
then cattle alone produce about 4.57 tons of oocysts per year in the U.S.!
 Fecal oocyst production in Holstein calves each
inoculated at
4 days of age with 25 million Cryptosporidiium parvum oocysts.
Each data point represents oocysts quantified from a 24 hr fecal collection
period.
Fecal oocyst production in Holstein calves each
inoculated at
4 days of age with 25 million Cryptosporidiium parvum oocysts.
Each data point represents oocysts quantified from a 24 hr fecal collection
period.
Cryptosporidium parvum appears to make little effort to evade the immune system of the host. Many of the surface proteins, glycoproteins, and phospholipids are strongly immunogenic, far more so than traditional enteric coccidia, and many molecules on the surface of both sporozoites and merozoites are antigenically cross-reactive. The success of the parasite appears to be in its ability to develop rapidly and flood the environment with oocysts. In fact, if this parasite were not efficiently eliminated from the body, it would quickly kill an animal through dehydration and electrolyte imbalance and rapidly eliminate host species from the environment. Thus, it seems plausible that the high immunologic profile may actually represent a survival strategy.
Enough surveys have been conducted to gain some idea about prevalence of the parasite in the environment. In industrialized nations, somewhere around 0.4% of the population appears to be passing oocysts in the feces at any one time. Of those patients admitted to hospitals for diarrhea, 2-3% are passing oocysts. However, the sero-prevalence is much higher and 30-35% (in one study over 50%) of the US population have antibodies to C. parvum. In third world countries, the sero-prevalence is even higher and up to 60-70% (in some studies up to 85%) of people in these countries may have circulating antibodies to this pathogen. Because recent studies have suggested serum antibodies to wane with time, it is likely that most adult humans have been infected with the parasite at least once in their lives. In AIDS patients, the numbers of individuals suffering from chronic cryptosporidiosis has been about 10% in industrialized nations and up to 40% in some third world countries. In the last few years, however, the use of proteinase inhibitors plus nucleoside analogs in AIDS patients seems to have lowered the actual numbers of AIDS patients suffering from chronic cryptosporidiosis in the US. Still, the only truly effective means of eliminating the parasite is a healthy, intact immune system.
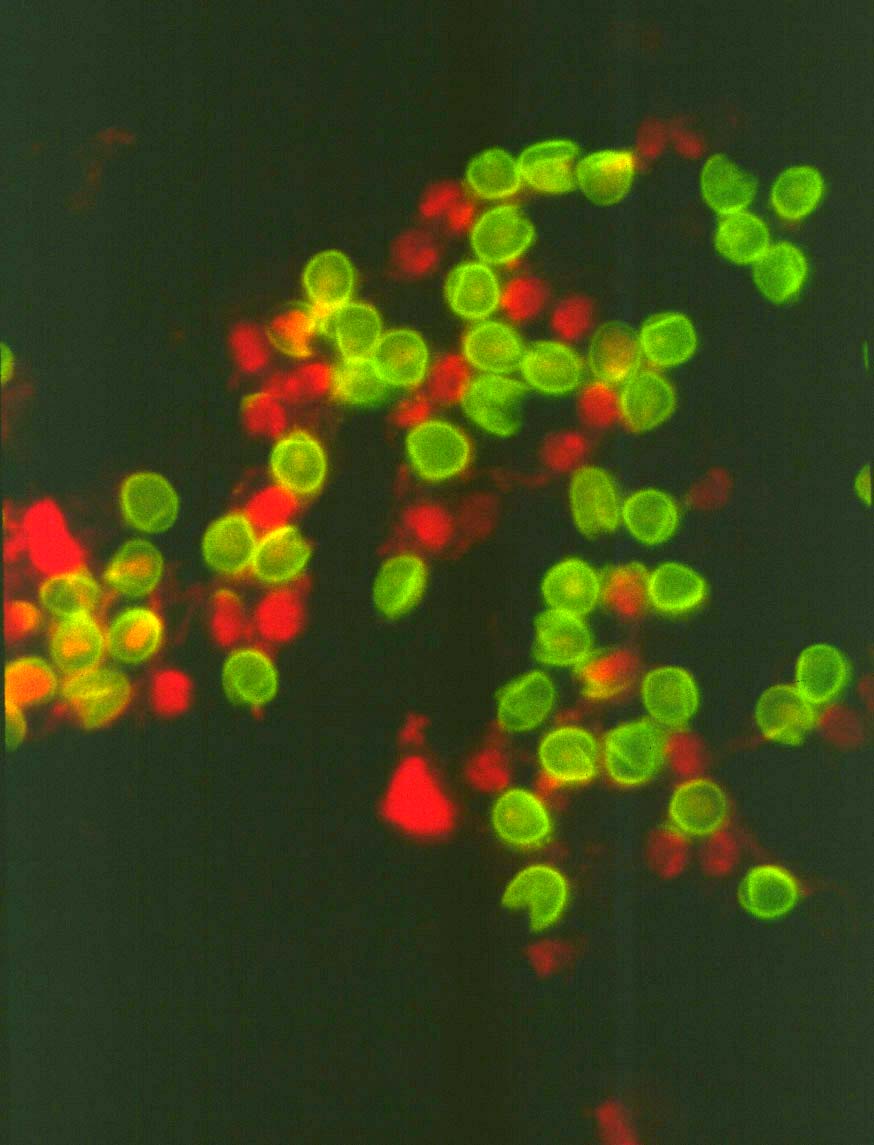
 Oocysts of Cryptosporidium parvum
labeled in an indirect immunofluorescence assay (IFA) with monoclonal
antibody 8F4 to the inner oocyst wall. The IFA is still the most commonly
used technique to assay for oocysts in environmental samples.
Oocysts of Cryptosporidium parvum
labeled in an indirect immunofluorescence assay (IFA) with monoclonal
antibody 8F4 to the inner oocyst wall. The IFA is still the most commonly
used technique to assay for oocysts in environmental samples.
Oocysts of Cryptosporidium are widespread in the environment and can be found in lakes and streams. The time of year when Cryptosporidium becomes a problem in surface waters in most areas of North America is generally March-June, when spring rains increase run-off and many neonate animals are present in the environment to amplify oocyst numbers. It should be noted, however, that studies are now showing that many adult animals continue to produce low levels of oocysts on a regular basis, which enhances the environmental load and serves as a source of infection for neonates. Ruminants, cervids, swine, cats, dogs, and other mammals may all contribute to numbers of Cryptosporidium oocysts in the environment both in rural and urban areas. Breakdowns or overloading of public water utilities have occasionally resulted in community outbreaks of cryptosporidiosis. In other cases, infections have been acquired from swimming pools and water parks because of fecal accidents. In most cases, various degrees of diarrhea, some weight loss and abdominal cramping were the extent of illness. In some individuals, however, specifically young children, the elderly, and immunosuppressed patients, cryptosporidiosis became chronic and life-threatening. It should be noted that it is nearly impossible to determine the origin of many individual cases of cryptosporidiosis. There are endless numbers of anecdotal reports of the parasite being acquired from public water supplies. Many of these may well represent cases of cryptosporidiosis transmitted to humans by companion animals such as kittens and puppies, from ruminants on farms, or by contact with other humans.
Recent studies have shown that Cryptosporidium "parvum" senso latu exists as no less than two distinct species. Genotype 1 (or genotype H for human) is now termed Cryptosporidium hominis and is almost exclusively a parasite of humans (with a few minor exceptions). Genotype 2 (or genotype C for calf) is considered the traditional Cryptosporidium parvum and occurs in a wide range of animals, including humans. The former species tends to be more aggressive in humans, with a patent period nearly doubling that of genotype 2 and averaging just under 2 weeks. Rarely, both species can be found infecting the same person. Genetic markers on different chromosomes reveal there is little or no mixing between the two (i.e. isolates are not found that are composed of mixed genotypes), strongly supporting the notion that two distinct (but morphologically identical) species exist. Either species may cause an outbreak, however, and all seven isolates typed (that I know of) during the 1993 Milwaukee outbreak were found to be Cryptosporidium hominis (genotype 1).
The intestine is a large place for a protozoan and no one really knows how many oocysts it takes to establish an infection in humans. One study suggested that the 50% infectious dose in humans was around 132 oocysts, although one volunteer was infected with as few as 30 oocysts. Another study using a more aggressive isolate suggests that even lower numbers of oocysts (nine) can sometimes initiate infections and cause disease (1999, J Inf Dis 180: 1275-1281). Humans, like animals, appear to have various degrees of susceptibility to this parasite and the effective doses will probably be shown to vary between individuals and among isolates. We do know that the numbers of Cryptosporidium oocysts reported by various groups from public water samples are highly inaccurate. Concentration techniques for oocysts in environmental samples are poor and detection methods often cross- react with algae or other debris. Numerous other species of Cryptosporidium incapable of infecting humans occur in the environment and may cross-react in diagnostic tests. In addition, many oocysts detected are probably not viable due to age, freezing, or UV radiation (in our laboratory, exposure to temperatures high enough to denature proteins, as well as freezing solid to -10 C, always kills the parasites). Finally, one recent report has shown quite clearly that the various laboratories performing diagnostic testing on Cryptosporidium oocysts in water have widely varying degrees of accuracy.
PREVENTION AND CONTROL. Because all Cryptosporidium infections are initiated though ingestion of environmentally resistent oocysts, control of this stage is the single most important factor in limiting the spread of the disease. Infected animals and humans will continue to contaminate the environment, and elimination of these sources is virtually impossible.
A variety of commercial disinfectants have been used in an attempt to kill sporozoites within oocysts. However, most of these have little or no effect on parasite infectivity even when C. parvum oocysts were exposed at intervals ranging from 30 min to 24 hr. At least some oocysts remain infective even after being exposed to 15,000 mW/sec UV light for 2 hr (but not 2.5 hr) (1993, J. Parasitol. 79: 67-70), -15 C for 24 hr (but not 1 wk) (1994, Appl. Environment. Microbiol. 60: 2732-2735), -20 C for 8 hr (but not 24 hr) (1994, Appl. Environment. Microbiol. 60: 2732-2735), and +59.7 C for 5 min (but not +60 C for 6 min) (1996, Appl. Environment. Microbiol. 62: 1431-1433). Typically, 100% of the encysted parasites are killed only when extreme and unpractical measures are employed. These include exposure to 1 J/sq cm pulsed light (1995, Food Technol. 49: 95-98), 100% bromomethane gas for 24 hr (1996, Appl. Environment. Microbiol. 62: 3908-3909), 28,000 mg/L chlorine for 24 hr (In, Cryptosporidium in Water Supplies, 1990, J. Badenoch ed., London), 10% formol saline for 18 hr (1982, Vet. Rec. 111: 414-415), 5% ammonia for 18 hr (1982, Vet. Rec. 111: 414-415), and 100% ethylene dioxide gas for 24 hr (1996, Appl. Environment. Microbiol. 62: 3908-3909). Lengthy exposures to gaseous or aqueous solutions of ammonia, hydrogen peroxide, high concentrations of chlorine and related compounds, and short term exposure to ozone, significantly reduce numbers of viable C. parvum oocysts but only rarely result in 100% efficacy.
Perhaps the single most effective and economical method of reducing the numbers of oocysts in the environment is simply dessication. Using dye exclusion to measure viability, Robertson et al. (1992, Appl. Environment. Microbiol. 58: 3494-3500) found viability of a population of air dried C. parvum oocysts to be reduced by 97% after 2 hr and 100% after 4 hr. Feces containing C. parvum oocysts and air dried for a single day was found to be non-infectious for suckling mice in another study (1986, Am. J. Vet. Res. 47: 2272-2273). These data imply that application of aqueous disinfectant solutions, which keep feces moist, may actually result in prolonging parasite survival in feces rather than reducing parasite numbers.
For more information on the general biology of Cryptosporidium and its occurrence in the environment, you may wish to examine the following general biology articles (arranged chronologically).
Current, W.L. and Reese, N.C. 1986. A comparison of endogenous development of three isolates of Cryptosporidium in suckling mice. Journal of Protozoology 33: 98-108. [complete life cycle presented both at the light and ultrastructural level].
Current, W.L., Upton, S.J., and Haynes, T.B. 1986. The life cycle of Cryptosporidium baileyi n. sp. (Apicomplexa, Cryptosporidiidae) infecting chickens. Journal of Protozoology 33: 289- 296. [Life cycle at both the light and ultrastructural level of the species pathogenic in poultry].
Fayer, R. and Ungar, B.L.P. 1986. Cryptosporidium spp. and Cryptosporidiosis. Microbiological Reviews 50: 458-483.
Current, W.L. 1988. The biology of Cryptosporidium. ASM News 54: 605-611. [This is a very simple and useful review suitable for both high school and undergraduate college courses].
Dubey, J.P., Speer, C.A., and Fayer, R. 1990. Cryptosporidiosis of Man and Animals. CRC Press, Boca Raton, Florida. 199 pp.
Casemore, D.P. 1990. Epidemiological aspects of human cryptosporidiosis. Epidemiology and Infection 104: 1-28.
Current, W.L. and Garcia, L.S. 1991. Cryptosporidiosis. Clinical Microbiology Reviews 4: 325-358.
Sterling, C.R. and Arrowood, M.J. 1992. Cryptosporidia. In, Parasitic Protozoa, 2nd edition, Volume 6. Edited by Kreier, J.P. Academic Press, NY. pp. 159-225.
Clancy, J.L. et al. 1994. Commercial labs: how accurate are they? J. Am. Water Works Assoc. 86: 89-97.
MacKenzie, W.R. et al. 1994. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. New England Journal of Medicine 331: 161-167. [describes the 1993 outbreak in Milwaukee, Wisconsin].
Millard, P.S. et al. 1994. An outbreak of cryptosporidiosis from fresh-pressed apple cider. Journal of the American Medical Association 272: 1592-1596. [you gotta read this one].
O'Donoghue, P.J. 1995. Cryptosporidium and cryptosporidiosis in Man and Animals. International Journal for Parasitology 25: 139-195.
MacKenzie, W.R. et al. 1995. Massive outbreak of waterborne Cryptosporidium infection in Milwaukee, Wisconsin. Recurrence of illness and risk of secondary transmission. Clin. Inf. Dis. 21: 57-62.
Addiss, D.G. et al. 1996. Reduction of risk of watery diarrhea with point-of-use water filters during a massive outbreak of waterborne Cryptosporidium infection in Milwaukee, Wisconsin, 1993. Am. J. Trop. Med. Hyg. 54: 549-553.
Fayer, R. 1997. Cryptosporidium and cryptosporidiosis. CRC Press, Boca Raton, FL. 251 pp.
Tzipori, S. and J.K. Griffiths. 1998. Natural history and biology of Cryptosporidium parvum. Adv. Parasitol. 40: 5-36.
Tzipori, S. and G. Widmer. 2000. The biology of Cryptosporidium. Contrib. Microbiol. 6: 1-32.
Fayer, R., U. Morgan, and S.J. Upton. 2000. Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 30: 1305-1322.
Duszynski, D.W. and S.J. Upton. 2001. Chapter 16. Enteric protozoans: Cyclospora, Eimeria, Isospora, and Cryptosporidium spp. In, Parasitic Diseases of Wild Mammals, 2nd edition. W.M. Samuel, M.J. Pybus, and A. Kocan, eds. Wildlife Disease Association, Iowa State University Press, Ames, Iowa. pp. 416-459.
Xiao, L., Fayer, R., Ryan, U., and Upton, S.J. 2003. Cryptosporidium taxonomy: recent advances and implications for public health. Crit. Rev. Microbiol. (in press).
Morgan-Ryan, U.M., Fall, A., Ward, L.A., Hijjawi, N., Sulaiman, I., Fayer, R., Thompson, R.C.A., Olson, M., Lal, A., and Xiao, L. 2002. Cryptosporidium hominis n. sp. (Apicomplexa: Cryptosporidiidae) from Homo sapiens. J. Euk. Microbiol. 49: 433-440.
Steve J. Upton, PhD
Division of Biology, Ackert Hall
Kansas State University
Manhattan, KS 66506

Home | Search | What's
New | Help | Comments
Kansas State University | Biology Division